Abstract
Background: Ruxolitinib (RUX) is a potent JAK1/JAK2 inhibitor and is effective in patients (pts) with myelofibrosis (MF). However, suboptimal or declining responses to RUX occur in a subset of pts, possibly due to persistent PI3K pathway activation with chronic JAK inhibitor therapy. Parsaclisib is a potent and highly selective next-generation PI3Kδ inhibitor. We conducted a phase 2 study (NCT02718300) evaluating add-on parsaclisib to stable doses of RUX, for pts with MF who experienced a suboptimal response to RUX (Yacoub A. HemaSphere 2021;5 Suppl 2:512). Here we report efficacy and safety results from the completed study.
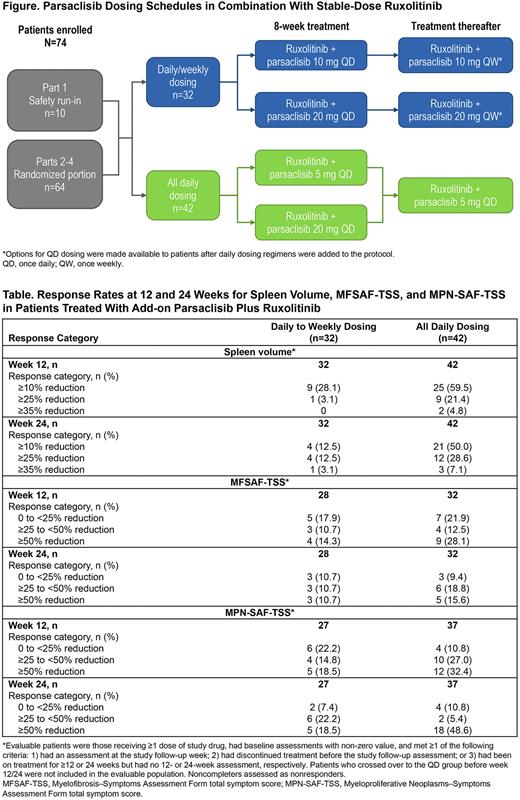
Methods: Eligible pts had primary or post-essential thrombocythemia/post-polycythemia vera MF with suboptimal response (palpable spleen >10 cm below left subcostal margin [LSM], or palpable splenomegaly 5-10 cm below LSM and presence of 1 symptom score ≥5 or 2 symptom scores ≥3 each using the Screening Symptom Form) after ≥6 months of RUX (5-25 mg twice daily; RUX stable dose, ≥8 wks). Pts remained on their stable RUX dose and were randomized to receive add-on parsaclisib 10 mg or 20 mg once daily (QD) for 8 wks and the same dose once weekly (QW) thereafter (QD/QW group), or parsaclisib 5 mg or 20 mg QD for 8 wks and 5 mg QD thereafter (all QD group) (Figure). Primary efficacy endpoint was change in spleen volume (SV) from baseline to wk 12 by MRI/CT scan. Key secondary endpoints included change in SV to wk 24, change in total symptom score (MFSAF-TSS, MPN-SAF-TSS), and safety.
Results: Overall, 32 pts received parsaclisib QD/QW and 42 pts received parsaclisib all QD. Baseline median age was 68.0 (range 41.0-89.0) years and 47.3% of pts were male. The study has been completed. Median treatment duration was 336.5 days; median average daily dose was 5.0 mg/day for parsaclisib and 29.8 mg/day for RUX. Among all pts, 16 (21.6%) were rolled over to continue therapy in an open-label parsaclisib study after ending treatment in the present study. Other common reasons for pts discontinuing treatment were adverse events (13 [17.6%]) and progressive disease (10 [13.5%]); remainder discontinued for physician decision, pt decision, or "Other." Baseline median SV (cm3) was 2414.5 in QD/QW (n=29) and 1877.5 in QD (n=37); median MFSAF-TSS was 10.8 (n=28) and 16.3 (n=32) and median MPN-SAF-TSS was 25.5 (n=28) and 30.0 (n=37), respectively.
Median percentage change in SV at wk 12 was −1.6 (n=28) in QD/QW and −15.4 (n=37) in QD, and at wk 24 was −2.5 (n=20) and −19.3 (n=29), respectively. Median percentage change in MFSAF-TSS at wk 12 was −14.0 (n=21) in QD/QW and −32.8 (n=24) in QD, and at wk 24 was −10.0 (n=16) and −44.4 (n=18), respectively. Median percentage change in MPN-SAF-TSS at wk 12 was −19.2 (n=20) in QD/QW and −39.8 (n=30) in QD, and at wk 24 was −43.3 (n=15) and −60.8 (n=26), respectively. Responder analysis for these 3 efficacy variables is shown in the Table.
Nonhematologic treatment-emergent adverse events (TEAEs) were primarily grade 1/2. Grade 3/4 nonhematologic TEAEs occurring in >1 pt overall were pneumonia (n=5; 3 QD/QW, 2 QD), fatigue, hypoxia (each n=2; each 1 QD/QW, 1 QD), dyspnea (n=2, all QD), fall, alanine aminotransferase (ALT) increased, aspartate aminotransferase (AST) increased, hypocalcemia (each n=2; all QD/QW). New-onset grade 3 thrombocytopenia was observed in 6/32 pts (18.8%) in QD/QW and 11/42 pts (26.2%) in QD; grade 4 thrombocytopenia was observed in 6/32 pts (18.8%) in QD/QW and 3/42 pts (7.1%) in QD. TEAEs of special interest included grade ≥3 ALT and AST (each n=2; all QD/QW), grade ≥2 diarrhea (n=4) and rash (n=1) (all QD/QW), and any-grade herpes simplex (n= 4; 1 QD/QW, 3 QD) and VZV infection (n= 3; 1 QD/QW, 2, QD). No colitis was reported. TEAEs led to parsaclisib interruption in 16/32 pts in QD/QW and 22/42 pts in QD, and RUX interruption in 5/32 and 8/42 pts, respectively. TEAEs led to parsaclisib discontinuation in 5/32 pts in QD/QW and 4/42 pts in QD, and RUX discontinuation in 2/32 and 2/42 pts, respectively.
Conclusion: Final results from the phase 2 study demonstrate improvement in symptoms and SV with add-on parsaclisib in patients with MF having a suboptimal response to ruxolitinib. All daily dosing regimens were more efficacious than daily/weekly dosing. Combination therapy was associated with limited grade 3/4 AEs and TEAE-related discontinuations. Phase 3 studies using 5 mg QD parsaclisib together with ruxolitinib in naive MF patients and ruxolitinib-experienced patients are in progress.
Disclosures
Yacoub:AbbVie: Consultancy; Agios: Consultancy; Servier: Consultancy; Stemline Therapeutics: Research Funding; Notable Labs: Consultancy; Gilead: Consultancy; Apellis: Consultancy; Acceleron Pharma: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; PharmaEssentia: Consultancy; CTI Pharma: Consultancy; Incyte: Consultancy, Speakers Bureau. Borate:Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie/Genentech: Membership on an entity's Board of Directors or advisory committees. Rampal:Zentalis: Consultancy, Research Funding; Novartis: Consultancy; Stemline: Consultancy, Research Funding; Celgene/BMS: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint: Consultancy; Stemline: Consultancy, Research Funding; Galecto: Consultancy, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Consultancy; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy; Incyte: Consultancy, Research Funding; Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI: Consultancy; Promedior: Consultancy; Gilead: Consultancy; Sierra Oncology: Consultancy; Disc Medicines: Consultancy; Sunimoto Dainippon: Consultancy. Ali:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Wang:AbbVie, Astellas, Daiichi Sankyo, Dava Oncology (Arog), Gilead, Genentech, Jazz Pharmaceuticals, Kite Pharmaceuticals, Kura Oncology, MacroGenics, Pfizer, PTC Therapeutics, Stemline Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie, Genentech, Rafael Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Other: Independent data review committees; Pfizer, Stemline Therapeutics: Other: Speaker. Gerds:CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Accurate Pharmaceuticals: Research Funding; Incyte Corporation: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imago BioSciences: Research Funding; Kratos Pharmaceuticals: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys/Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees. Hobbs:Bayer: Research Funding; Pharmaxis: Other: Advisor or review panel participant; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Abbvie Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Constellation: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; PI, Research Funding; Pfizer: Other: Advisor or review panel participant; Incyte: Other: Advisor or review panel participant; PI, Research Funding; Bristol Myers Squibb Co./Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Keros: Other: Advisor or review panel participant; Merck: Research Funding. Kremyanskaya:BMS: Research Funding; Protagonist Therapeutics: Consultancy, Research Funding; Kura: Research Funding; Ionis: Research Funding; Incyte: Consultancy, Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Research Funding; Chimerix: Research Funding; Kronos: Research Funding. Winton:Blueprint Medicines Corporation, Samus Therapeutics and Incyte Corporation: Research Funding. O'Connell:Astex Pharmaceuticals, Genentech: Research Funding; Astex Pharmaceuticals, Bristol Myers Squibb, Pfizer, Shionogi: Membership on an entity's Board of Directors or advisory committees. Oh:Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Disc Medicine: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgne/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees. Schiller:Cellerant: Research Funding; Incyte: Other: speaker fees, Research Funding, Speakers Bureau; Deltafly: Research Funding; Amgen: Current equity holder in publicly-traded company, Honoraria; Daiichi-Sankyo: Research Funding; Cyclacel: Research Funding; Jazz: Consultancy; Kite, a Gilead Company: Research Funding, Speakers Bureau; Stemline: Research Funding; PreCOG LLC: Research Funding; Trovagen: Research Funding; Mateon: Research Funding; Medimmune: Research Funding; Janssen: Research Funding; Novartis: Honoraria, Other: Speaker fees, Research Funding; AstraZeneca: Honoraria; Actuate: Research Funding; Arog: Research Funding; Constellation: Research Funding; Deciphera: Research Funding; Cellectis: Research Funding; Forma: Research Funding; Agios: Consultancy, Honoraria; Onconova: Research Funding; Ono Pharma: Honoraria; CTI: Research Funding; Stemline: Speakers Bureau; Astellas: Research Funding, Speakers Bureau; Millennium: Research Funding; Pfizer: Research Funding; Regimmune: Research Funding; Geron: Research Funding; Bristol Myers Squibb: Current equity holder in publicly-traded company, Speakers Bureau; Johnson & Johnson: Current equity holder in publicly-traded company; FujiFilm: Research Funding; AltruBio: Research Funding; Celgene: Consultancy, Research Funding, Speakers Bureau; Karyopharm: Research Funding, Speakers Bureau; Glycomimetics: Research Funding; AVM Biopharma: Research Funding; Genentech-Roche: Research Funding; Samus: Research Funding; Gamida: Research Funding; Actinium: Research Funding; Sellas: Research Funding; Gilead: Research Funding; AbbVie: Research Funding, Speakers Bureau; Sangamo: Research Funding; Takeda: Research Funding; Tolero: Research Funding. Assad:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Erickson-Viitanen:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Zhou:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Daver:Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding.
OffLabel Disclosure:
Parsaclisib is an investigational drug being assessed in combination with ruxolitinib for patients with myelofibrosis
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal